Background: The goal of clinical trial eligibility criteria is to identify a population of patients that have high risk disease or have unmet needs with standard of care therapy. Frontline trials of diffuse large B-cell lymphoma (DLBCL) include patients with International Prognostic Index (IPI) scores of 3-5 as a way to identify a high risk population. Trials typically include patients with IPI score=2, and sometimes IPI score=1, to expand the eligible patient population. However, broader patient populations must be balanced by the potential inclusion of low-risk patients with excellent outcomes to standard of care therapy, leading to potentially underpowered trials. IPI is a sum of dichotomous variables which makes implementation easy but can result in heterogeneity in outcomes within IPI scores. This heterogeneity may be caused by unequal prognostic information across IPI variables, loss of information within IPI variables due to the use of cutoffs, as well as information captured by variables that are not included in the IPI. In prior modeling of outcomes in DLBCL (Maurer et al, AJH 2016), we identified that: i) LDH has a strong prognostic ability beyond the upper limit of normal (ULN) and ii) bulky disease is an independent variable for prognosis. Here we evaluate if the presence of either of these two variables, easily captured at diagnosis within the current standard clinical work-up, can identify a high-risk subset of patients within the population of those with IPI scores of 1-2.
Methods: Patients with newly diagnosed lymphoma were enrolled within 6 months of diagnosis in the LEO Cohort at 8 academic medical centers from 2015-2020 and prospectively followed. Inclusion criteria for this analysis was aggressive B-cell lymphoma typically included in frontline trials (e.g. DLBCL, high grade B-cell lymphoma, FL3B), IPI 1-5, frontline therapy with rituximab and anthracycline-based chemotherapy, stage II-IV disease and ages 18-80 years. Event-free survival (EFS) was defined as the time from diagnosis until progression, initiation of 2 nd line therapy, or death due to any cause; EFS was evaluated at 24 months (EFS24). Very high LDH was defined as LDH>1.3xULN based on prior modeling (Maurer et al, AJH 2016) and bulky disease was defined as maximum tumor diameter (MTD) of 7cm or greater. IPI 1 and 2 were first evaluated separately and then combined due to similar outcomes in patients with very high LDH and/or bulky disease. High risk IPI1-2 was defined as the presence of either bulky disease and/or very high LDH within an IPI score of 1-2. Confirmation was performed in a similar cohort of patients enrolled in the Iowa/Mayo SPORE Molecular Epidemiology Resource (MER) from 2002-2015; bulky disease was defined using a 10cm cutoff per availability in MER data collection forms.
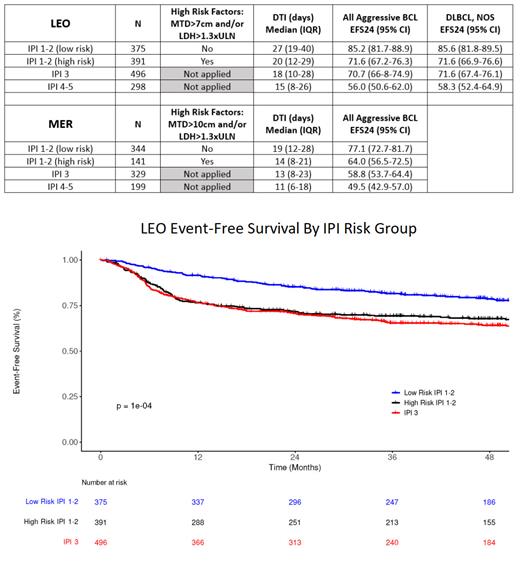
Results: 1560 patients from LEO were analyzed; median age was 64 years (IQR: 54-71), 42% were female and 83% had stage III/IV disease; subtype was DLBCL, NOS in 1335 (86%). IPI was 1-2 in 766 patients, 3 in 496 patients and 4-5 in 298 patients ( table). 391 (51%) with IPI1-2 had either bulky disease and/or very high LDH (high risk IPI1-2) and 375 had low-risk IPI1-2. EFS24 in high risk IPI1-2 (71.6%, 95% CI: 67.2-76.3) was significantly inferior to IPI1-2 without bulky disease or very high LDH (85.2, 95% CI: 81.7-88.9 logrank p=0.0035, figure) and similar to patients with IPI3 (70.7%, 95% CI: 66.8-74.9). Consistent results were observed in 1014 patients analyzed in the MER using a higher cutoff for bulky disease (10cm) due to data availability ( table). Patients with high risk IPI1-2 had significantly shorter diagnosis to treatment interval (DTI) in both the LEO (per-week OR=0.83, 95% CI: 0.77-0.88) and MER (per-week OR=0.77, 95% CI: 0.68-0.87) cohorts compared to low risk IPI1-2, both p<0.0001). In sensitivity analysis, LEO results were similar when performed in the subset of patients with DLBCL, NOS.
Conclusion: The presence of either bulky disease and/or very high LDH at presentation identifies a high-risk subset of patients with IPI 1-2 that have similar outcomes to patients with IPI 3 disease. These simple criteria can be easily implemented within existing clinical trial diagnostic work-ups. Future trial designs in frontline aggressive B-cell lymphoma should include patients with IPI 3-5 and patients with IPI 1-2 with either bulky disease and/or very high LDH to better define a high-risk population of patients for clinical trial eligibility.
Disclosures
Maurer:BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Farooq:MorphoSys: Consultancy; Kite, a Gilead Company: Honoraria; Caribou: Consultancy, Honoraria; Regeneron: Research Funding. Romancik:KITE: Consultancy; Astra Zeneca: Consultancy. Lossos:NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy. Kahl:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; ADCT: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Lilly: Consultancy, Honoraria. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Habermann:BMS: Research Funding; Genentech: Research Funding; sorrento: Research Funding. Cerhan:Genmab: Research Funding; Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding. Flowers:Genentech Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Celgene: Consultancy, Research Funding; V Foundation: Research Funding; Sanofi: Research Funding; Kite: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Research Funding; Pfizer: Research Funding; National Cancer Institute: Research Funding; Adaptimmune: Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Xencor: Research Funding; SeaGen: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Burroghs Wellcome Fund: Research Funding; Ziopharm: Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; Pharmacyclics Jansen: Consultancy; Jannsen Pharmaceuticals: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Abbvie: Consultancy, Research Funding; Novartis: Research Funding; Denovo Biopharma: Consultancy; 4D: Research Funding; Acerta: Research Funding; Iovance: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Spectrum: Consultancy; Takeda: Research Funding; Beigene: Consultancy; Bayer: Consultancy, Research Funding. Nastoupil:Daiichi Sankyo: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Regeneron: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Nowakowski:Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bantam Pharmaceutical LLC: Consultancy; ADC Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy; Kymera Therapeutics: Consultancy; MEI Pharma: Consultancy; Seagen: Consultancy; Selvita Inc: Consultancy; Zai Lab Limited: Consultancy; Curis: Consultancy; Abbvie: Consultancy; TG Therapeutics: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal